Tag: bioequivalence
Long-term studies show generics are generally as safe as brand-name drugs, but key differences emerge for high-risk medications and certain manufacturers. Learn when switching could affect your health and what to ask your pharmacist.
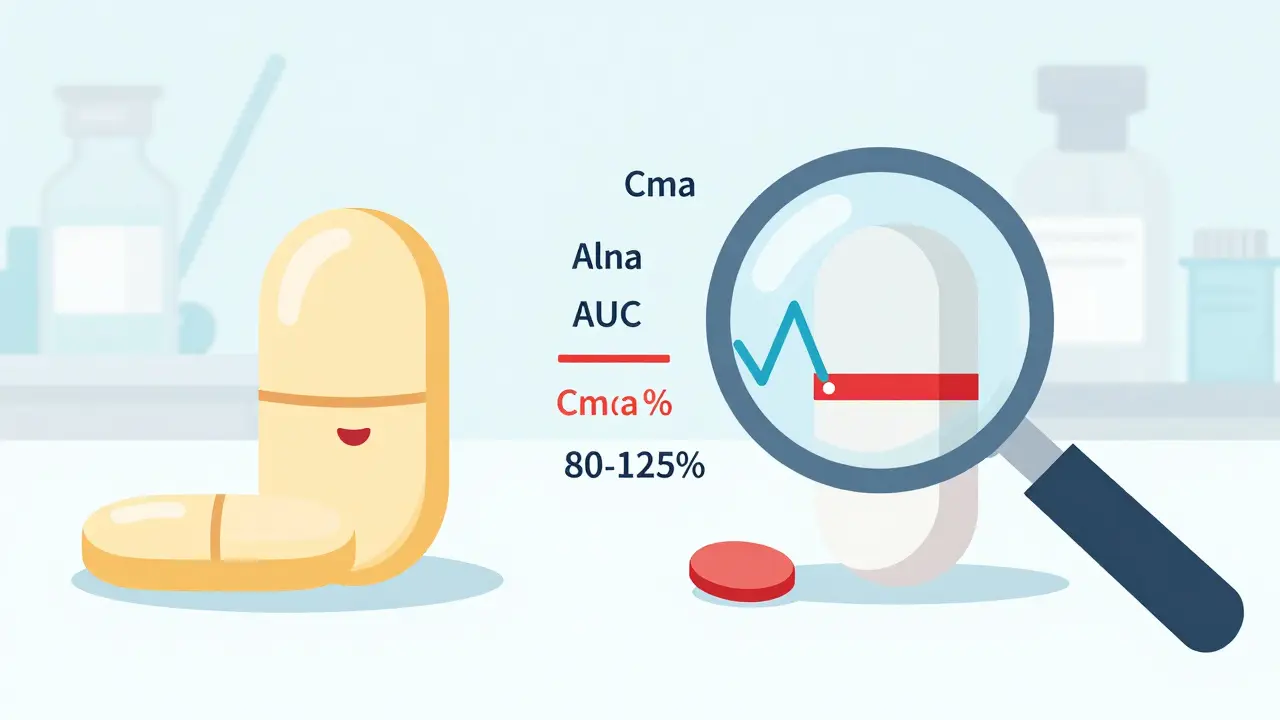
Bioequivalence standards for generic drugs have long ignored batch-to-batch variability, risking false conclusions. New guidelines now require multi-batch testing to ensure real-world equivalence and patient safety.
Generic drugs work just like brand-name ones but cost up to 85% less. Learn how the FDA ensures they're safe, when to choose them, and why they save billions every year.
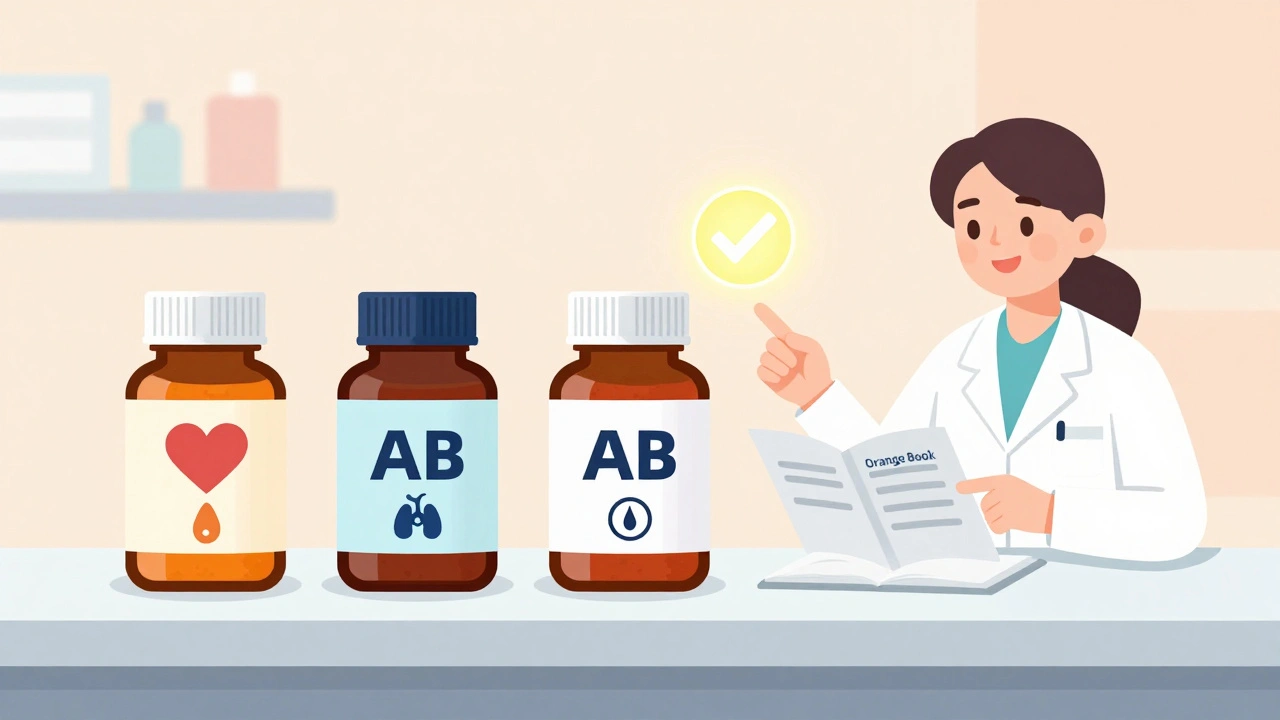
Learn how to safely choose between multiple generic medications by understanding therapeutic equivalence ratings, bioequivalence, and when to stick with one manufacturer. Avoid risks with narrow therapeutic index drugs like warfarin and levothyroxine.
False advertising in generic drugs misleads patients about safety, equivalence, and cost. Learn the legal risks, FDA rules, and how deceptive ads can harm health and trigger lawsuits.





 Medications
Medications




