When you pick up a prescription, you might see two names on the label: one you recognize, like Lipitor, and another you don’t, like atorvastatin. The first is the brand-name drug. The second is the generic. They’re the same medicine - but one costs a fraction of the other. So why does the price difference exist? And should you care?
They’re not different medicines - just different labels
Generic drugs aren’t cheaper because they’re weaker or made with lower-quality ingredients. They’re cheaper because they don’t have to repeat the same expensive clinical trials that brand-name drugs do. By law, a generic drug must contain the same active ingredient, in the same strength, and work the same way in your body as the brand-name version. The U.S. Food and Drug Administration (FDA) requires every generic to prove it’s bioequivalent - meaning it delivers the same amount of active ingredient into your bloodstream at the same rate as the brand-name drug. That’s not a guess. It’s a strict test. The acceptable range? 80% to 125% of the brand’s absorption. For high-risk drugs like warfarin or levothyroxine, the range is even tighter: 90% to 111%.Why do brand-name drugs cost so much?
Brand-name drug companies spend years and hundreds of millions of dollars developing a new medication. They run clinical trials, pay for regulatory reviews, and market the drug heavily. All of that gets built into the price. Once the patent expires - usually after 20 years from filing, though the effective market exclusivity is often 12-14 years - other companies can make the same drug. They don’t need to redo the trials. They just need to prove their version works the same way. That cuts their costs dramatically. In the U.S., generics cost 80-85% less than their brand-name counterparts. That’s not a small difference. On average, a prescription for a generic drug costs $12.50 with insurance. The brand-name version? Around $68.30. For someone taking medication every day for years - like high blood pressure or cholesterol pills - that adds up to thousands saved per year.What’s different about generics?
The only differences between a generic and its brand-name twin are the things that don’t affect how the drug works: color, shape, size, flavor, and inactive ingredients like fillers or dyes. These are changed to avoid trademark infringement. A pill can be blue instead of yellow, oval instead of round, and still be identical in how it treats your condition. Packaging might look different too. But the active ingredient? Exactly the same. Some people worry about the inactive ingredients. For most, it’s not an issue. But if you have a rare allergy to a dye or preservative, you should check the label. Your pharmacist can tell you what’s in each version.
When generics might not be the best choice
For the vast majority of medications - over 90% of prescriptions - generics work just as well. But there are exceptions. Some drugs have what’s called a narrow therapeutic index. That means even a tiny change in blood levels can cause problems. Warfarin (a blood thinner), levothyroxine (for thyroid issues), and certain seizure medications fall into this category. Studies show that even for these drugs, generic versions are generally safe. A 2019 analysis of 38,000 patients on levothyroxine found no difference in outcomes between brand and generic. But some doctors and patients still prefer sticking with one version - brand or generic - to avoid any possible fluctuation. If you’re on one of these drugs and you switch from brand to generic, your doctor might want to monitor your levels more closely at first. There’s also the issue of availability. About 30% of brand-name drugs still don’t have generic versions because the patent hasn’t expired, or the drug is too complex to copy - like inhalers, injectables, or topical creams. The FDA is working on speeding up approval for these complex generics, with 150 potential candidates identified in 2023.What patients actually experience
Real-world feedback is mostly positive. A 2022 Consumer Reports survey of over 1,200 people found that 89% believed generics were just as effective as brand-name drugs. On Reddit’s r/Pharmacy community, 86% of users reported no difference after switching from brand to generic for common drugs like lisinopril, metformin, and atorvastatin. But 14% did report issues. One user shared that their mother’s seizure control worsened after switching from brand Lamictal to generic lamotrigine. She had to go back to the brand. Another person said their asthma symptoms returned after switching from Advair to the generic version. These cases are rare, but they happen. Often, the problem isn’t the drug itself - it’s how the body reacts to a change in formulation, even if it’s technically equivalent. If you notice a change in how you feel after switching - more side effects, less effectiveness - talk to your doctor. Don’t assume it’s all in your head. Your experience matters.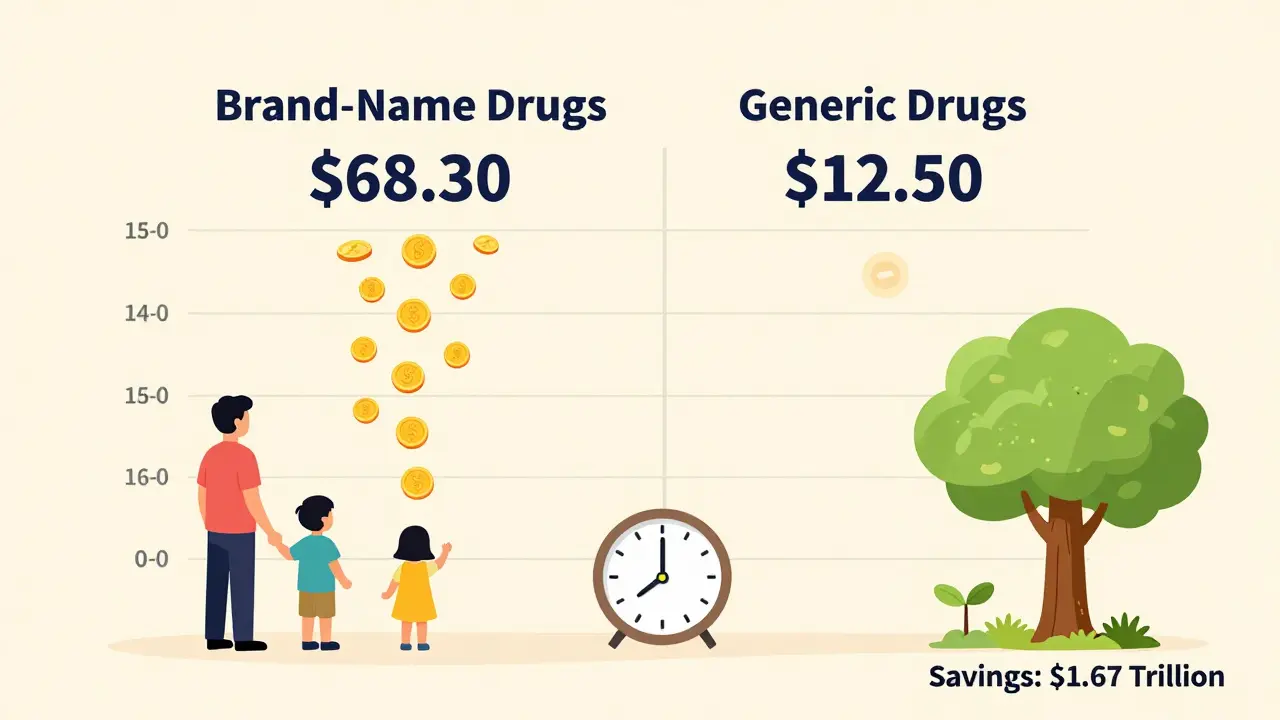
How to make sure you get the right version
In 49 states, pharmacists are legally required to substitute a generic if one is available - unless your doctor writes “dispense as written” on the prescription. That means if you don’t say otherwise, you’ll get the cheaper version. To avoid confusion, keep a simple list of your medications with both the brand and generic names. If your doctor switches you from brand to generic, write it down. If you refill and the pill looks different, check with your pharmacist before taking it. A small change in appearance doesn’t mean it’s the wrong drug - but it’s good to confirm. Also, don’t assume all generics are the same. Different manufacturers might make slightly different versions. If you switch between two generics and notice a difference, stick with the one that works best for you.The bigger picture: why generics matter
Generics aren’t just good for your wallet - they’re good for the whole system. In the U.S., generics make up 90% of prescriptions but only 25% of drug spending. That means for every dollar spent on medicine, 75 cents goes to brand-name drugs, even though you’re getting generics for most of your pills. From 2007 to 2016, generics saved the U.S. healthcare system $1.67 trillion. That’s billions every year. The Inflation Reduction Act of 2022 and new FDA rules are pushing for faster approval of generics, especially for high-cost drugs. By 2028, over 450 more brand-name drugs will lose patent protection, opening the door for cheaper alternatives. The goal? Lower costs, more access, and better health outcomes for everyone.Bottom line: generics are safe, effective, and worth choosing
There’s no reason to avoid generics unless you’ve personally noticed a problem - and even then, it’s usually fixable. The FDA, the American Medical Association, and top pharmacists all agree: generics are not second-rate. They’re the same medicine, tested just as rigorously, at a fraction of the cost. If you’re on a long-term medication - for diabetes, high blood pressure, cholesterol, or depression - ask your doctor or pharmacist if a generic is available. You could save hundreds, even thousands, without sacrificing your health. Don’t let the name on the bottle scare you. What matters is what’s inside - and that’s exactly the same.Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for safety, strength, quality, purity, and effectiveness as brand-name drugs. Every generic must prove it works the same way in your body before it can be sold. There’s no difference in safety between the two.
Why do generic pills look different?
U.S. trademark laws prevent generic drugs from looking exactly like brand-name versions. That’s why the color, shape, or size might be different. But the active ingredient - the part that treats your condition - is identical. The differences are only in inactive ingredients like dyes or fillers, which don’t affect how the drug works.
Can I switch between different generic versions?
Yes, but if you’re taking a drug with a narrow therapeutic index - like warfarin or levothyroxine - it’s best to stick with one manufacturer. Switching between generics might cause small changes in how your body absorbs the drug. If you notice any side effects or changes in how you feel after switching, talk to your doctor.
Why doesn’t every drug have a generic version?
Some drugs are still under patent protection, which gives the original maker exclusive rights to sell it. Others are too complex to copy easily - like inhalers, biologics, or certain topical creams. About 30% of brand-name drugs don’t have generics yet. But more are becoming available every year as patents expire.
Will my insurance cover generics?
Yes, and they usually cost much less. Most insurance plans have lower copays for generics. Some even require you to use the generic version before approving the brand-name one. Always check your plan’s formulary - it’s the list of covered drugs - to see what you’ll pay.
What should I do if I think the generic isn’t working?
Don’t stop taking it. Contact your doctor or pharmacist right away. Sometimes, changes in how you feel are due to other factors - stress, diet, or other medications. But if you notice a real difference in effectiveness or side effects after switching to a generic, your doctor can help you decide whether to switch back or try a different generic manufacturer.



 Medications
Medications

![Buy Generic Zovirax (Acyclovir) Online Cheap in the UK [2025]: Safe, Legal, and Fast](/uploads/2025/08/thumbnail-buy-generic-zovirax-acyclovir-online-cheap-in-the-uk-2025-safe-legal-and-fast.webp)

Russ Kelemen
January 31, 2026 AT 19:16It’s wild how much we trust labels over science. I used to think generics were ‘second-tier’ until I started tracking my own meds. Switched from brand-name metformin to generic-same pill, same results, $80/month saved. My bank account thanked me more than my doctor did.
And honestly? The FDA doesn’t cut corners. If they say it’s bioequivalent, it is. We’re not talking ‘close enough’-we’re talking statistically identical absorption curves. That’s not magic. That’s rigorous science.
April Allen
February 1, 2026 AT 02:30The bioequivalence range of 80–125% is statistically robust, but it’s worth noting that for drugs with narrow therapeutic indices, even minor inter-individual variability in Cmax or AUC can tip the scales-especially in polypharmacy patients. Pharmacokinetic modeling shows that switching between generics can introduce cumulative absorption variance, which is why some clinicians prefer brand consistency in anticoagulants or antiepileptics.
That said, the overwhelming majority of patients experience no clinically significant difference. The fear is often rooted in confirmation bias, not pharmacology.
Kathleen Riley
February 1, 2026 AT 16:10One must consider the epistemological implications of pharmaceutical commodification. The very notion that a molecule, identical in composition, can be rendered ‘inferior’ by its packaging and pricing structure speaks to a deeper societal pathology: the fetishization of brand over substance. We have elevated corporate logos to the status of divine sigils, while the material reality-the active pharmaceutical ingredient-remains, in all its banal equivalence, utterly ignored.
Beth Cooper
February 2, 2026 AT 08:13Wait-so you’re telling me the government says generics are the same… but why do I feel weird after switching? I think they’re hiding something. I read online that the fillers in generics are laced with microchips to track us. And don’t get me started on the dye-they’re using it to manipulate our moods. My cousin’s dog got sick after eating a generic pill. Coincidence? I think not.
Also, why do all the pills look different? That’s not science. That’s control.
Melissa Cogswell
February 3, 2026 AT 09:29I’ve been on levothyroxine for 12 years. Switched to generic last year after my insurance forced it. My TSH stayed perfectly in range. No symptoms changed. My pharmacist even showed me the FDA bioequivalence data-it’s actually reassuring. If you’re worried, ask for the manufacturer name on the bottle. Stick with one if it works. No need to overthink it.
Bobbi Van Riet
February 5, 2026 AT 04:53I get why people are nervous. I was too. My mom’s on warfarin, and when they switched her to generic, she swore her bruises got worse. We went back to brand for a month-she felt better. Then we tried a different generic manufacturer-same as the brand, different pill shape-and she was fine. Turns out, it wasn’t the drug-it was the change. Our bodies hate change, even when it’s not needed.
So if you switch and feel off? Don’t panic. Talk to your doc. Try a different generic. Maybe your body just needs time. But don’t assume it’s broken. Most of the time, it’s not.
Sazzy De
February 6, 2026 AT 05:05generic works fine
save money
no need to stress
Rohit Kumar
February 7, 2026 AT 13:01In India, generics are the norm-not because we’re poor, but because we’re smart. We don’t pay for marketing hype. We pay for science. My father took generic atorvastatin for 10 years. His cholesterol stayed stable. His bank account stayed full. The FDA standards are global. If it passes in the U.S., it passes everywhere. Trust the data, not the logo.
Jodi Olson
February 9, 2026 AT 02:46Generics are just as good. The FDA doesn’t lie. The only thing that changes is the color and the price. Everything else? Identical. If you’re not seeing results, it’s not the pill. It’s something else. Don’t blame the science. Blame the noise.
Carolyn Whitehead
February 9, 2026 AT 16:19I used to be scared to switch too but then I started reading the studies and talking to my pharmacist and honestly it’s been fine. My anxiety about it was way worse than any side effect. Sometimes we make things harder than they need to be. Just let the science do its thing
Beth Beltway
February 10, 2026 AT 20:48Oh please. You all act like the FDA is some saintly guardian of truth. They’re a regulatory body that’s been captured by Big Pharma. The 80-125% bioequivalence window is a joke. That’s a 45% variance! That’s not ‘equivalent’-that’s ‘maybe works, maybe doesn’t.’ And don’t get me started on the fact that 30% of generics are made in China and India with sketchy oversight. You think they’re testing every batch? Please. You’re a lab rat in a corporate experiment.
And if you’re okay with that, you’re not brave-you’re naive.
Natasha Plebani
February 11, 2026 AT 02:58There’s a critical nuance here that’s often lost: bioequivalence is population-level, not individual-level. A drug may meet FDA thresholds across a cohort, yet exhibit pharmacodynamic variability in specific genotypes-CYP2C9 polymorphisms, for instance, can alter warfarin metabolism. This doesn’t invalidate generics-it just means precision medicine isn’t one-size-fits-all.
For most, generics are perfectly adequate. For the 1–3% with idiosyncratic metabolism or severe hypersensitivity, individualized monitoring is warranted. The system isn’t broken. It just needs better stratification.