When you pick up your prescription and see three different boxes on the counter - all with the same drug name but different brand logos - it’s easy to feel confused. Generic medication isn’t just cheaper. It’s supposed to work the same. But not all generics are created equal. And when you’re taking something for your heart, thyroid, or epilepsy, even small differences matter.
What Makes a Generic Drug Really the Same?
The U.S. Food and Drug Administration (FDA) doesn’t just approve any copycat pill. Every generic must prove it delivers the same active ingredient, in the same strength, and the same way into your body as the brand-name version. That’s called pharmaceutical equivalence. But that’s only half the story.
The real test is bioequivalence. This means the generic must release the drug into your bloodstream at a rate and amount that falls within 80% to 125% of the brand-name drug’s performance. That might sound like a big range - but in practice, most approved generics are within 5% of the original. A 2017 FDA analysis showed the average difference in blood levels between generics and brand-name drugs was just 3.5%.
Here’s the myth you need to bust: Generics don’t contain 20% less active ingredient. That’s a common misunderstanding. The 80-125% range applies to how fast and how much of the drug enters your blood - not how much is in the pill. The actual amount of active ingredient is identical.
Therapeutic Equivalence Ratings: The Secret Code on the Label
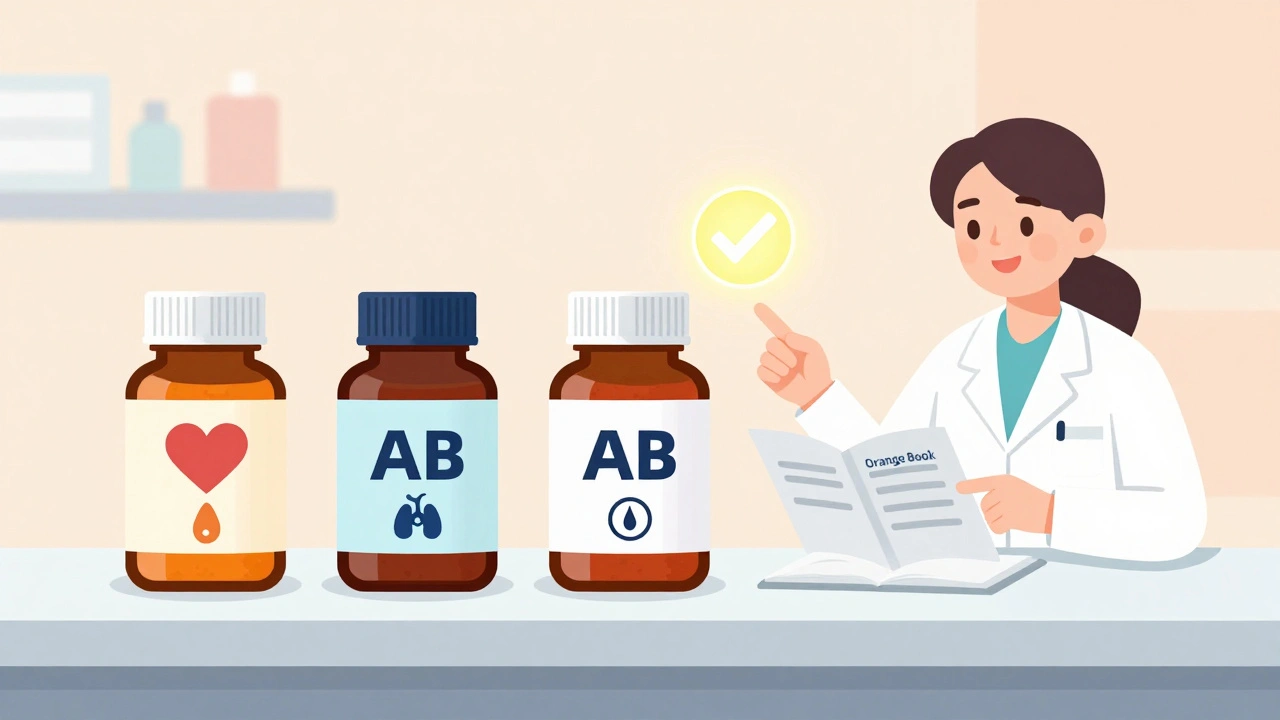
The FDA doesn’t just say a generic is approved. It gives it a letter code - and this is what you need to look for. It’s in the Orange Book, a public database that lists every approved drug and its rating.
Two ratings matter most:
- AB - This means the generic has been proven bioequivalent and is considered fully interchangeable with the brand. You can switch between AB-rated generics without concern.
- B - These generics meet FDA standards, but there’s evidence they behave differently in the body. They’re not unsafe, but they may not be ideal for switching if you’re already stable on another version.
For example, if you’re taking a generic version of levothyroxine (for hypothyroidism) and your doctor switches you to a different generic with a B rating, your thyroid levels could shift. That’s not because the drug is bad - it’s because your body has adapted to a specific formulation.
When Switching Generics Can Be Risky
Not all drugs are equal when it comes to switching. Some have what’s called a narrow therapeutic index. That means the difference between the right dose and a dangerous one is tiny.
These drugs need extra care:
- Warfarin (blood thinner) - Even a 10% change in blood levels can increase your risk of clotting or bleeding.
- Levothyroxine (thyroid hormone) - Your body is sensitive to tiny fluctuations. Studies show switching brands can cause TSH levels to rise or fall.
- Digoxin (heart medication) - A 2017 study in Circulation found that some generic versions caused more side effects in the first month after switching.
- Phenytoin (anti-seizure) - Small changes can trigger seizures.
The Endocrine Society, the American Heart Association, and the FDA all agree: once you’re stable on a specific version - brand or generic - stick with it. Don’t switch unless you have to.
Why Do Different Generics Behave Differently?
If the active ingredient is the same, why do some generics work differently?
It’s the inactive ingredients. Fillers, coatings, binders, and release mechanisms vary between manufacturers. For most drugs, these don’t matter. But for time-release pills, or drugs absorbed in specific parts of the gut, these differences can change how quickly or completely the drug enters your system.
Take extended-release metformin. One generic might release the drug slowly over 12 hours. Another might release it faster. Both are approved. But if you’re used to the slow one and switch, you might get stomach upset or feel the effect too soon.
That’s why the FDA now requires more detailed testing for complex generics - like inhalers, patches, or modified-release tablets. Since 2022, they’ve published over 1,000 product-specific guidelines to make sure these versions are truly equivalent.
How to Choose Between Multiple Generics
If you’re starting a new medication, you have more flexibility. But if you’re switching from a brand or from one generic to another, follow these steps:
- Check the Orange Book - Ask your pharmacist for the therapeutic equivalence rating. Look for AB-rated versions.
- Don’t switch unless necessary - If you’re stable on a brand or generic, stay on it. Frequent switching increases risk.
- Ask for the same manufacturer - Even among AB-rated generics, one company’s version might work better for you. If you’ve had no issues with a particular generic, ask your pharmacist to keep filling that one.
- Watch for changes - After switching, pay attention to side effects, energy levels, or symptoms returning. Keep a simple log: date, dose, how you felt.
- Talk to your doctor - If you notice changes, don’t assume it’s ‘all in your head.’ Bring your log. Ask: ‘Could this be the generic?’
For new patients: B-rated generics are often cheaper. If you’re starting a medication and cost is a big factor, a B-rated version may be fine - as long as your doctor monitors you closely.
What Pharmacists Can Do for You
Your pharmacist is your best ally in this process. They see every generic on the market. They know which ones are AB-rated. They can tell you if a switch will require a new prescription or if they’re legally allowed to substitute.
In 28 U.S. states, pharmacists must notify your doctor if they switch you to a different manufacturer’s generic than the one you were previously taking. In other states, they can switch without telling you - which is why you need to ask.
Don’t be shy. Say: ‘I’m on this generic. Can you make sure the next refill is the same one?’ Or: ‘I had side effects after switching. Can we stick with this brand?’
Pharmacists can also help you find the lowest-cost AB-rated option. Sometimes, two generics have the same rating, but one is $10 cheaper. That’s the one to pick - if it’s AB-rated.
What’s Changing in 2025?
The FDA is pushing for better tracking. A proposed law called the Generic Drug Labeling Act would require each manufacturer’s version to have its own unique barcode (NDC code). Right now, many generics share the same code - making it hard to trace which one caused a problem.
That’s a big step forward. It means in the future, if you have a bad reaction, your doctor and pharmacist can tell exactly which version you took - not just the drug name.
Also, the FDA is now requiring stricter testing for complex generics like inhalers and topical creams. These used to be approved with less data. Now, they must prove they work the same way in the body - not just in a lab.
Bottom Line: Stay Consistent, Stay Informed
Generic drugs save the U.S. healthcare system over $370 billion a year. That’s huge. And for most people, switching to a generic is safe and smart.
But if you’re taking a drug where small changes matter - heart, thyroid, seizure, or blood thinner - consistency is your safety net. Don’t let cost alone decide your medication. Let your body’s response and your doctor’s advice guide you.
When in doubt, ask: Is this AB-rated? Is this the same manufacturer as before? Should I watch for changes? Three simple questions can keep you safe.
Are generic medications as safe as brand-name drugs?
Yes, FDA-approved generic medications are required to meet the same safety and effectiveness standards as brand-name drugs. They contain the same active ingredient, in the same strength and dosage form. The FDA monitors adverse events for both types of drugs and has found no consistent difference in safety profiles for most medications. However, for drugs with a narrow therapeutic index - like warfarin or levothyroxine - switching between different generic versions can sometimes cause changes in blood levels, which is why consistency is key.
Can I switch between different generic versions of the same drug?
For most medications, yes - especially if they’re AB-rated. But for drugs with a narrow therapeutic index (like digoxin, levothyroxine, or phenytoin), frequent switching between generics is not recommended. Even small differences in how the drug is absorbed can affect your health. If you’re stable on one version, stick with it. If you must switch, monitor for symptoms and check your blood levels if your doctor recommends it.
What does an AB rating mean on a generic drug?
An AB rating from the FDA means the generic drug has been proven bioequivalent to the brand-name version. It has the same active ingredient, strength, dosage form, and route of administration, and it delivers the drug into your bloodstream at the same rate and extent. AB-rated generics are considered fully interchangeable with the brand and are the safest choice for switching.
Why are some generics cheaper than others?
Price differences come from manufacturing costs, competition, and how long a company has been producing the drug. The first generic to enter the market after a brand expires often charges more. As more manufacturers enter, prices drop. Two generics with the same AB rating can differ in price by 50% or more. But price doesn’t indicate quality - always check the therapeutic equivalence rating, not the cost.
Should I always choose the cheapest generic?
Only if it’s AB-rated and you’re not taking a drug with a narrow therapeutic index. For most medications, the cheapest AB-rated generic is the best choice. But for drugs like warfarin, thyroid hormones, or anti-seizure medications, sticking with the same manufacturer - even if it’s slightly more expensive - can prevent dangerous fluctuations in your blood levels. Cost savings shouldn’t come at the risk of your health.
Can I ask my pharmacist to give me the same generic every time?
Absolutely. You have the right to request the same generic manufacturer every time. Pharmacists are required to honor your preference if you ask. Say: ‘I’ve been taking this specific generic and it works well for me - can you keep filling this one?’ If they say they can’t, ask them to contact your doctor. Many states require pharmacies to notify prescribers when switching manufacturers, so your doctor can decide if it’s safe.
What should I do if I feel different after switching to a new generic?
Don’t ignore it. Keep a simple log: note the date you switched, the name of the new generic (check the label), and any changes in how you feel - fatigue, dizziness, nausea, or return of symptoms. Bring this to your doctor. They may order a blood test to check drug levels (for drugs like warfarin or levothyroxine) or adjust your dose. You’re not imagining it - small changes in absorption can make a real difference.
What to Do Next
Take five minutes today. Look at your last prescription bottle. Find the name of the generic drug and the manufacturer. Check if it’s AB-rated. If you’re not sure, call your pharmacist. Ask: ‘Is this the same version I’ve been taking? Is it AB-rated?’
If you’re on a high-risk medication, ask your doctor if you should stick with one version - brand or generic - long-term. Don’t wait for a problem to happen. Prevention is simple: know what you’re taking, and don’t let it change without you knowing.


 Medications
Medications




val kendra
December 3, 2025 AT 17:36Just switched my levothyroxine to a cheaper generic and felt like a zombie for two weeks. Turned out the new one was B-rated. My endocrinologist was pissed I didn’t ask first. Don’t be me. Check the Orange Book.
Karl Barrett
December 4, 2025 AT 17:43It’s fascinating how the FDA’s 80-125% bioequivalence window is statistically sound but biologically nuanced. The active ingredient is identical, sure - but the excipients? They’re not inert. They’re pharmacokinetic conductors. For drugs with narrow therapeutic indices, that’s not a bug - it’s a feature of biological individuality. We treat pills like they’re interchangeable widgets, but the body doesn’t care about cost centers. It cares about absorption curves, dissolution rates, and gut flora interactions. The real issue isn’t generics - it’s the illusion of interchangeability in complex pharmacology.
Ben Choy
December 5, 2025 AT 15:26My grandma’s on warfarin and they switched her generic last month. INR went from 2.4 to 4.1. She nearly bled out. Now we only get the exact same brand - even if it’s $15 more. Worth every penny. 🙏
Emmanuel Peter
December 6, 2025 AT 12:39People are so dramatic about generics. If you can’t handle a 5% variance in blood levels, maybe you shouldn’t be on meds at all. It’s not rocket science. Just take the damn cheap one and stop whining. Your insurance company is paying for your panic.
Jenny Rogers
December 8, 2025 AT 08:01It is a moral failing of the American pharmaceutical system that patients must become pharmacologists simply to avoid iatrogenic harm. The FDA’s regulatory framework, while ostensibly robust, has been systematically undermined by corporate lobbying and the commodification of human physiology. One must not only know the Orange Book - one must interrogate its limitations. This is not healthcare. It is pharmacological roulette.
Scott van Haastrecht
December 8, 2025 AT 19:20Oh here we go again. Another ‘careful with generics’ lecture. I’ve been on 7 different generic versions of metformin and I’m still alive. Your fearmongering is what’s killing people - not the pills. Stop being a hypochondriac and get over it. Also, your pharmacist isn’t your therapist.
Heidi Thomas
December 9, 2025 AT 03:28Stop overthinking it. If the generic is AB-rated it’s the same. If you feel weird after switching, it’s probably stress or coffee. I’ve switched 12 times and never had an issue. People make this way too complicated. Just take the cheapest one and move on.
Libby Rees
December 10, 2025 AT 13:12My father has been on digoxin for 15 years. He switched generics twice - both times, he developed atrial fibrillation. The third time, we insisted on the original manufacturer. His rhythm normalized within days. This isn’t theory. It’s lived experience. Ask your pharmacist for the manufacturer name. Write it down. Don’t assume.
Dematteo Lasonya
December 11, 2025 AT 14:56My sister has epilepsy and got switched to a new generic phenytoin last year. Seizures came back. She didn’t say anything at first because she didn’t want to be ‘that patient.’ I made her log everything - dates, doses, symptoms. We went back to her doctor. Turns out the new one was B-rated. Now she gets the same one every time. It’s not expensive. It’s just not automatic. Ask. Always ask.
Rudy Van den Boogaert
December 13, 2025 AT 03:33My pharmacist told me the same thing: stick with the same maker. I didn’t even know I could ask. Now I say ‘same as last time’ every time I pick up. Saved me from a bad reaction on my blood pressure med. Easy fix, zero cost. Why doesn’t everyone know this?
Jordan Wall
December 14, 2025 AT 15:53Look, I’m a biochemist and I’ve reviewed the FDA’s 2022 product-specific guidelines for modified-release formulations - the data is messy. Most AB-rated generics are fine, but for complex delivery systems like transdermal patches or extended-release capsules, the bioequivalence studies are often underpowered. The system is a facade. We’re playing Russian roulette with excipients. And don’t get me started on the NDC code chaos 😅