Drug Risk-Benefit Calculator
Understand what drug labels really mean by converting relative risk reduction to absolute risk reduction. This tool helps you see how many people actually benefit from a medication.
Benefit-Risk Calculator
Your Results
If 100 people took this drug: - people would benefit compared to placebo.
How this works:
Relative risk reduction shows the percentage decrease compared to the placebo group, while absolute risk reduction shows the actual difference between treatment and placebo groups. This tool converts one to the other using the formula:
Absolute risk reduction = Baseline risk × (Relative risk reduction / 100)
When you pick up a new prescription, the tiny print on the drug label might feel like a foreign language. You see numbers, medical terms, and long paragraphs about side effects-but what does it all really mean for you? The U.S. Food and Drug Administration (FDA) spends years weighing whether a drug’s benefits outweigh its risks. But translating that complex decision into something patients can understand? That’s where things get messy.
What the FDA Actually Does
The FDA doesn’t just approve drugs because they work. They approve them only if the benefits are strong enough to justify the risks. For example, a cancer drug that shrinks tumors but causes severe nausea might still get approved because for someone with advanced cancer, living another six months matters more than feeling sick. But how do they decide this? The FDA uses a structured tool called the Benefit-Risk Framework, introduced in 2021. It’s not a formula. It’s a conversation. Reviewers look at four things: the disease being treated, what other treatments exist, how well the drug works, and how dangerous its side effects are.
Every approved drug’s label must include an integrated summary of benefits and risks. That’s required by law. But here’s the problem: most of that summary is written for doctors, not patients. It’s full of phrases like “statistically significant improvement in progression-free survival” or “incidence of serious adverse events was 12.3%.” Patients don’t know what that means. A 2022 survey found only 22% of patients felt confident they understood their drug’s risk-benefit balance. For those with lower health literacy, that number dropped to 9%.
Where the Labels Fall Short
Take a common antidepressant. The label might say: “In clinical trials, patients experienced a 40% greater reduction in depressive symptoms compared to placebo.” Sounds good, right? But 40% what? That’s a relative number. The real story? Out of 100 people, 30 improved with the drug. Out of 100 on placebo, 21 improved. That’s a 9-point difference-not 40. Patients hear “40% better” and think it’s a miracle. It’s not. This kind of misleading math happens often.
Another issue? Risk numbers are rarely put in context. A label might say: “1 in 1,000 patients developed liver damage.” But without knowing how that compares to other drugs or the natural risk of liver damage from the disease itself, that number means nothing. One patient might panic. Another might ignore it. Neither is better informed.
And then there’s the silence. Many labels for psychiatric, chronic pain, or autoimmune drugs don’t give numbers at all. They say things like “some patients experienced mood changes” or “fatigue was reported.” No frequencies. No comparisons. No way to weigh it.
The Good Examples
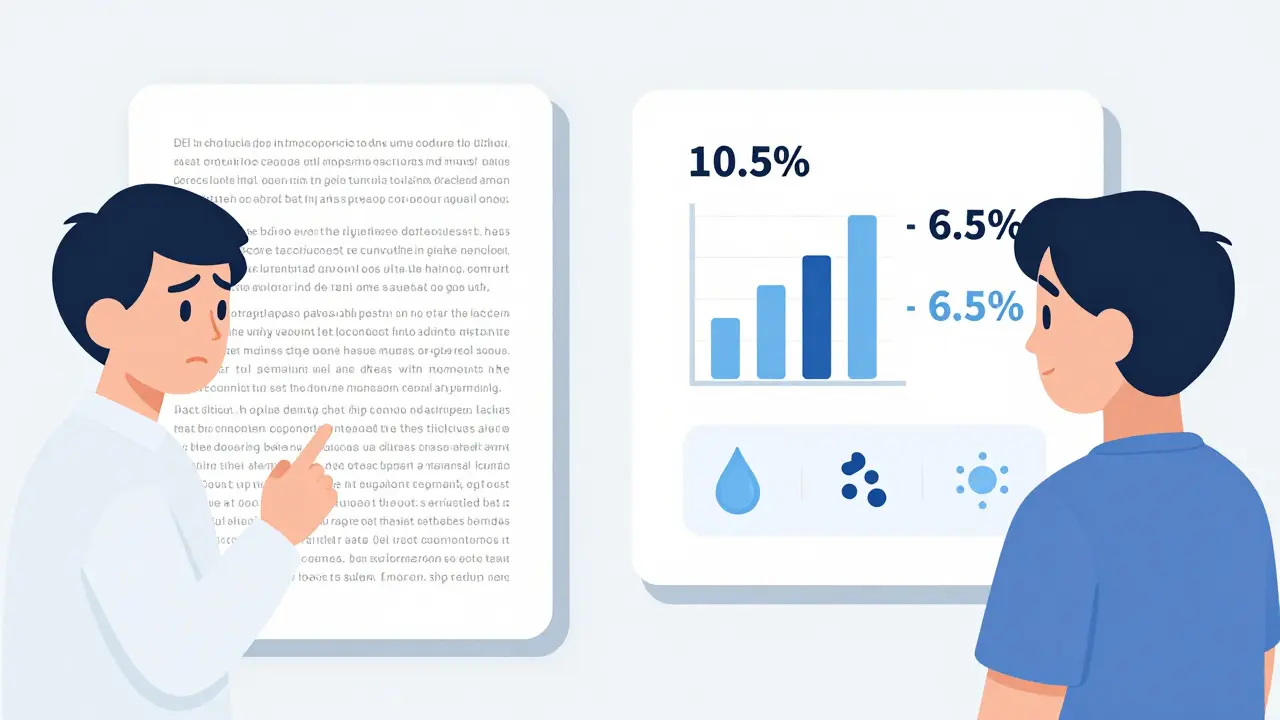
Not all labels are confusing. Some companies and the FDA itself have started getting it right. Take Jardiance, a diabetes drug for people with heart disease. Its label says clearly: “In adults with type 2 diabetes and cardiovascular disease, JARDIANCE reduced the risk of cardiovascular death by 38% (10.5% with placebo vs. 6.5% with JARDIANCE).” That’s concrete. You can picture it. Ten out of 100 people on placebo died from heart problems. Six and a half out of 100 on Jardiance did. That’s a real difference. Patients can understand that.
Another winner: the label for a new HIV medication that includes a simple graphic showing how much it lowers viral load compared to older drugs. No jargon. Just bars. One bar for the old drug. A taller bar for the new one. The side effects? Listed as small icons: one for nausea, one for dizziness, one for rash. You can see at a glance what you’re trading.
These aren’t accidents. They’re the result of patient feedback. In 2021, the FDA collected over 1,200 comments from patients. Nearly 80% asked for clearer comparisons to other treatments. Over 60% wanted visual tools. So the FDA started testing something new: Benefit-Risk Icons. Simple pictograms-like a heart for cardiovascular benefit, a skull for serious risk-that show relative size. Bigger icon = bigger impact. These are now being tested in 12 clinics with 1,500 patients.
Why It’s So Hard to Get Right
Here’s the truth: patients aren’t one group. One person might risk a 1% chance of stroke to avoid daily headaches. Another might refuse the same drug because they’re terrified of any side effect. The FDA has to make a decision for the average patient. But real decisions are personal.
Also, the science isn’t always clear. For drugs treating Alzheimer’s or depression, benefits are hard to measure. Is feeling “a little less sad” a benefit? Is slower memory loss a win? There’s no blood test for those. So labels rely on vague language. And vague language leaves patients confused.
Plus, the process is slow. FDA reviewers spend 40 to 60 hours on each drug just to write the benefit-risk summary. That’s a lot of time. And most companies don’t have staff trained in plain-language communication. Only 17% of new drugs approved in 2022 included any visual benefit-risk summary. That’s changing-but slowly.
What You Can Do
You don’t have to guess. Here’s how to read your label smarter:
- Look for numbers with context. If it says “reduced risk by X%,” ask: “X% compared to what?” Look for the placebo or control group numbers. They’re usually in Section 14.
- Check the absolute numbers. “1 in 100” is more meaningful than “90% reduction.” Ask your pharmacist: “How many people out of 100 actually got better? How many had a serious side effect?”
- Compare to alternatives. Ask your doctor: “What’s the benefit and risk of this drug vs. the next one?” Don’t accept “it’s the best option” without numbers.
- Ask for visuals. If your label has no charts or icons, ask if there’s a patient-friendly version online. The FDA’s website has plain-language summaries for many drugs.
- Use the Patient-Focused Drug Development site. The FDA has a public database where patients submit their experiences. Search your drug name there. You’ll find real stories from people who’ve taken it.
The Future Is Coming
The FDA is moving fast. By 2025, they plan to require standardized benefit-risk metrics for major drug categories-like heart disease, diabetes, and mental health. By 2026, nearly half of all new drug labels are expected to include visual summaries. That’s up from just 8% in 2022.
They’re also testing a new rule: every breakthrough therapy (the ones for life-threatening diseases) must include a Patient Benefit-Risk Summary written at a 6th-grade reading level. No jargon. Just facts, numbers, and simple pictures.
It’s not perfect. But it’s progress. And it’s happening because patients spoke up. Your voice matters. If a label feels confusing, tell your doctor. Tell your pharmacist. Tell the FDA. There’s now a public way to do it-and they’re listening.
Why do FDA drug labels use such complicated language?
FDA labels are written to meet legal and scientific standards, not to be easy to read. The language is designed for healthcare professionals who need precise, detailed data for prescribing. But that doesn’t mean it should stay that way. The FDA now recognizes that patients need clearer information, and they’re gradually shifting toward plain language-especially for new drugs approved after 2023.
Can I trust the benefit numbers on my drug label?
Yes-but only if you look at the full picture. Drug companies test their products in controlled trials, and the FDA reviews all the data. But numbers like “50% reduction in risk” are often relative. Always ask for the absolute numbers: “Out of 100 people, how many improved?” and “How many had serious side effects?” That’s the real measure of benefit.
Are there better examples of patient-friendly labels?
Yes. Drugs like Jardiance (for diabetes and heart disease), Ozempic (for weight loss and diabetes), and some newer cancer treatments now include clear statements with actual numbers: “Reduced risk of heart death from 10.5% to 6.5%.” Some also use simple icons to show benefit size vs. risk. These are becoming more common, especially in drugs approved after 2023.
What should I do if my drug’s label has no numbers?
Ask your doctor or pharmacist for the clinical trial data. You can also search the FDA’s website for the full prescribing information, which includes detailed tables. Look for Section 14 (Clinical Studies) and Section 6 (Adverse Reactions). If you’re still unsure, request a patient-friendly summary from the drug manufacturer-they’re required to provide one upon request.
Will all drug labels become easier to understand soon?
Not overnight, but yes-faster than before. By 2025, the FDA plans to require standardized benefit-risk metrics for major drug classes. By 2026, over 40% of new drug labels are expected to include visual summaries. This shift is being driven by patient demand and new FDA pilot programs. The change is coming, and it’s already started.



 Medications
Medications




Rachidi Toupé GAGNON
February 12, 2026 AT 22:34Vamsi Krishna
February 13, 2026 AT 13:24Brad Ralph
February 14, 2026 AT 15:27christian jon
February 15, 2026 AT 10:37Craig Staszak
February 16, 2026 AT 09:26Joanne Tan
February 16, 2026 AT 13:53Jack Havard
February 17, 2026 AT 01:00Autumn Frankart
February 18, 2026 AT 23:33Pat Mun
February 19, 2026 AT 11:51Sophia Nelson
February 20, 2026 AT 21:27Steve DESTIVELLE
February 21, 2026 AT 02:07Stephon Devereux
February 22, 2026 AT 14:00steve sunio
February 23, 2026 AT 22:39