Doctors prescribe generic drugs every day - but do they really understand them? In 2023, 90% of all prescriptions filled in the U.S. were for generics. Yet, many physicians still hesitate. Why? Because misinformation lingers. Patients ask: “Is this really the same?” And some doctors, despite years of training, aren’t fully equipped to answer.
What Makes a Generic Drug the Same as a Brand Name?
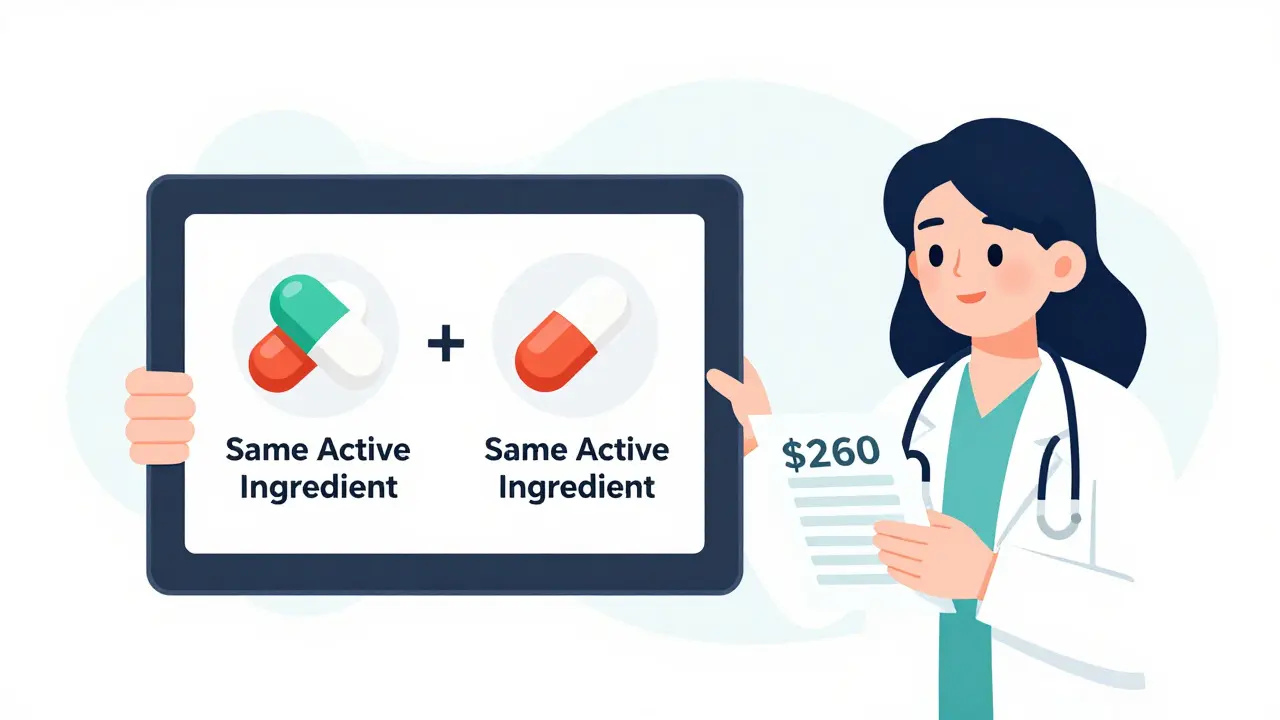
It’s not magic. It’s science. The FDA requires every generic drug to prove it delivers the same active ingredient, in the same amount, at the same rate as the brand-name version. This is called bioequivalence. The standard? The generic must fall within an 80% to 125% range of the brand’s absorption in the bloodstream. That’s not a guess - it’s measured in clinical trials with 24 to 36 healthy volunteers.
That means if a brand-name statin lowers cholesterol by 35%, the generic will do the same - within a tiny margin of error. The inactive ingredients? Those can differ. But they don’t affect how the drug works. Think of it like two identical cars with different paint jobs. Same engine. Same performance. Different color.
The FDA doesn’t approve generics based on cost. They approve them based on data. Every batch is tested. Every factory is inspected. And the same quality controls apply to both brand and generic manufacturers.
Why Do Doctors Still Doubt Generics?
It’s not about the science. It’s about perception. Some doctors grew up being told brand-name drugs were “better.” Others saw patients react poorly after switching - but those reactions were rarely due to the drug itself. More often, it was the placebo effect, or a change in pill size, color, or even the brand name on the bottle.
A 2023 survey of 1,247 physicians found that 68% thought FDA prescriber flyers were useful - but too technical for quick use during a 10-minute visit. One family doctor in Nebraska, Dr. Sarah Chen, turned things around by using a simple infographic: “Here’s how the FDA checks every generic before it hits the shelf.” She showed patients photos of the same lab equipment used for both brand and generic drugs. Her generic prescribing rate jumped from 62% to 89% in 18 months.
Another barrier? Time. A 2022 study in Annals of Internal Medicine found that 73% of doctors said they simply didn’t have time to look up resources during appointments. That’s why embedding this info directly into electronic health records (EHRs) is changing the game.
Where to Find Trusted, Practical Resources
The FDA’s Generic Drugs Stakeholder Toolkit is the gold standard. It’s free, updated regularly, and designed for real-world use. Inside, you’ll find:
- 12 ready-to-use social media templates to share with patients
- 5 customizable information cards you can print and hand out
- 3 infographics, including one that breaks down the 80-125% bioequivalence range visually
- QR codes linking to Spanish-language materials - critical for health equity
There’s also the Prescriber Flyer - a single-page, front-and-back reference that fits in any clinic waiting room rack. Version 2 (released March 2022) includes real patient scripts: “My insurance wants me to switch, but my doctor said the brand works better.” The flyer gives you a clear, evidence-based reply.
And for complex cases - like inhalers, topical creams, or injectables - the FDA’s Generic Drugs and Health Equity Handout addresses the extra concerns. These aren’t simple pills. Their delivery matters. But even here, data shows no meaningful difference in outcomes when generics are properly formulated.

Cost Isn’t Just a Patient Issue - It’s a Clinical One
Dr. Aaron Kesselheim from Harvard put it plainly: “For a $300/month brand-name drug, switching to generic saves the patient $262.50 every month - and the therapeutic effect is 99.7% identical.”
That’s not a small saving. It’s life-changing for someone choosing between medication and rent. The American College of Physicians found that 20-30% of new prescriptions go unfilled because of cost. Generics cut that number in half.
And it’s not just patients. A 2022 AMA survey showed doctors who received structured education on generics were 2.3 times more likely to start cost conversations with patients. That’s not just good medicine - it’s good practice. Patients trust doctors who help them navigate affordability without compromising care.
What’s Missing - and What’s Coming
Here’s the gap: Only 37% of major EHR systems (like Epic or Cerner) automatically pop up generic education tools during prescribing. That’s like having a GPS that doesn’t warn you about traffic.
Kaiser Permanente fixed this. They integrated FDA-approved generic facts directly into their Epic system. When a doctor typed in a brand-name drug, a small alert appeared: “A generic is available. 99.7% bioequivalent. Saves patient $260/month.” Within six months, brand-name prescribing dropped by 18.7%.
Now, the FDA is piloting an API that connects generic drug data directly to EHRs. Early results? A 15.2% increase in generic prescribing among participating doctors in just six months.
And AI is stepping in. IBM Watson Health tested a prototype that analyzed patient records - age, income, past refusals - then generated personalized messages for doctors to use. In a trial with 120 physicians, patient acceptance of generics jumped by 29 percentage points.

How to Start Using These Resources Today
You don’t need a grant or a team. You just need five minutes.
- Download the FDA Prescriber Flyer - print one, keep it on your desk. Use it as a quick reference.
- Print one information card - put it next to your prescription pad. When a patient asks, “Is this safe?”, hand it to them.
- Use the script - “The FDA requires generics to meet the same standards as brand names. The only difference? The price.”
- Ask your EHR vendor - “Do you have FDA generic education pop-ups?” If not, push for it.
- Track your own rate - At the end of each month, note how many generics you prescribed. Compare it to last month. You’ll be surprised how fast it grows.
One rural family physician in Nebraska didn’t wait for a system upgrade. She used a printer, a stapler, and the FDA’s infographic. Within a year, her patients were asking for generics - not because she pushed them, but because she explained them.
Why This Matters More Than Ever
From 2010 to 2020, generics saved the U.S. healthcare system $2.29 trillion. The next five years? Another $1.87 trillion is on the line. That’s not just money. It’s access. It’s adherence. It’s lives.
And with new patent expirations coming - dozens of high-cost drugs set to go generic in 2025 and 2026 - the pressure to get this right is rising. CMS now requires electronic prescribing for controlled substances. 44 states have laws mandating generic substitution. But if doctors don’t understand why it’s safe, they won’t prescribe it.
The tools are here. The data is clear. The cost savings are real. The only thing missing is action.
Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires every generic drug to prove it delivers the same active ingredient, in the same amount, and at the same rate as the brand-name version. This is called bioequivalence, and it’s measured through strict clinical testing. Generics must fall within an 80-125% range of the brand’s absorption in the bloodstream - meaning they work the same way in the body. Over 90% of prescriptions filled in the U.S. are for generics, and safety data shows no difference in outcomes.
Why do some patients think generics don’t work as well?
It’s often about perception, not science. Patients may notice differences in pill color, shape, or size - or even the brand name on the bottle - and assume that means the drug is different. Some reactions are due to the placebo effect. Others happen when switching from a brand to a generic with slightly different inactive ingredients, which can rarely affect absorption in sensitive patients. But these cases are uncommon. The FDA’s education materials include scripts to help doctors explain this clearly: “The medicine inside is identical. Only the outside changed.”
Can I trust generics made overseas?
Yes. The FDA inspects all manufacturing facilities - whether they’re in the U.S., India, China, or elsewhere - using the same standards. Every batch of generic drug must meet the same quality, purity, and potency requirements as brand-name drugs. In fact, many brand-name drugs are made in the same factories as generics. The location doesn’t determine safety - the inspection process does. The FDA conducts over 3,500 inspections annually on generic drug plants worldwide.
Are there cases where I shouldn’t prescribe a generic?
Rarely. For most drugs - including statins, antibiotics, blood pressure meds, and diabetes drugs - generics are equally effective. Exceptions exist for drugs with narrow therapeutic windows, like warfarin or levothyroxine, where small changes in absorption can matter. Even then, studies show most patients can switch safely with proper monitoring. The FDA and clinical guidelines recommend generics as first-line unless there’s documented, repeatable failure with a generic version. If a patient has had a clear negative reaction to a specific generic, then avoid that one - but don’t assume all generics are the same.
How can I get generic education resources into my EHR system?
Start by asking your EHR vendor if they support FDA generic drug alerts. If not, request it. Many systems - like Epic and Cerner - now offer integration with FDA data via API. Kaiser Permanente successfully added these alerts in 2021, reducing brand-name prescribing by nearly 20%. You can also download the FDA’s toolkit and create your own custom pop-up messages using your system’s template tools. Even a simple pop-up saying “Generic available. Saves patient $260/month.” can change prescribing behavior.
Do generics have the same side effects as brand-name drugs?
Yes. Because they contain the same active ingredient, side effects are identical. The FDA analyzes adverse event reports for both brand and generic drugs - and in 2022, there were nearly equal numbers reported: 12,467 for generics versus 11,832 for brand-name drugs. The difference? Cost. Generics reduce financial stress, which can actually lower the risk of side effects caused by non-adherence or missed doses. Patients who can’t afford their meds often stop taking them - leading to worse outcomes than any side effect.
What’s the difference between a generic and an authorized generic?
An authorized generic is the exact same drug as the brand-name version - same active ingredient, same manufacturer, same factory - just sold under a generic label and at a lower price. The brand company licenses it to a generic maker. Many doctors don’t realize this exists, and 61% of surveyed physicians were confused by it. The key point: if you see an authorized generic, it’s not a “different” version - it’s the brand, just cheaper. No switching risk. No uncertainty.
Is there data showing that educating doctors improves generic prescribing?
Yes. A 2022 randomized trial with 347 physicians found that just 22 minutes of focused education on bioequivalence and cost savings increased generic prescribing by 31%. Doctors who received training were 2.3 times more likely to initiate cost conversations with patients. The American Medical Association’s 2022 survey confirmed this: physicians with structured education prescribed generics at significantly higher rates. The data is clear: knowledge drives action.
Doctors don’t need more paperwork. They need clear, trusted tools - and the time to use them. The FDA, CDC, and leading medical groups have built those tools. Now it’s up to each of us to use them - not just for cost savings, but for better care, better outcomes, and better trust between patients and providers.


 Medications
Medications




Ian Long
January 7, 2026 AT 13:23Look, I don’t care what the FDA says - I’ve seen patients crash after switching to generics. One guy went from stable to seizures on the generic levothyroxine. No, it’s not ‘placebo.’ It’s chemistry. The body isn’t a calculator. And yeah, I get the cost savings, but I’m not gonna gamble with someone’s life just to save $260 a month.
Alicia Hasö
January 7, 2026 AT 21:00This is the most important conversation in modern medicine - and it’s being buried under bureaucracy and bias. 🙌
Doctors, we are the gatekeepers. When we hesitate, patients suffer. When we educate, they thrive.
I printed the FDA infographic. I laminated it. I keep it next to my stethoscope. Last week, a 72-year-old woman hugged me because she could finally afford her blood pressure med. That’s not a statistic - that’s why we became doctors.
Stop waiting for the system to fix itself. Grab the flyer. Use the script. Change a life. Today.
And yes - the science is solid. The data is overwhelming. The only thing holding us back? Our own outdated assumptions.
Chris Kauwe
January 8, 2026 AT 04:13Let’s deconstruct the bioequivalence fallacy. The 80-125% Cmax/AUC range is statistically permissible, not biologically optimal. Pharmacokinetic variance is non-linear, especially in polypharmacy populations. The FDA’s model assumes homogenous metabolic profiles - a gross oversimplification of human physiology. Moreover, the manufacturing variance between Indian and U.S. facilities is not audited with equal rigor - despite the FDA’s PR campaigns.
And let’s not ignore the regulatory capture: Big Pharma funds the very CROs that validate generics. Conflict of interest? No. Just ‘market efficiency.’
This isn’t about science. It’s about systemic coercion disguised as cost containment. We’re medical professionals, not insurance adjusters.
Meghan Hammack
January 9, 2026 AT 15:46My patient Maria came in crying because she couldn’t afford her brand-name insulin. I handed her the FDA card. Told her, ‘Same medicine. Different color. Same result.’ She cried harder - but this time, it was relief.
That’s it. That’s the whole thing.
No jargon. No charts. Just a simple truth: You’re not losing anything. You’re gaining everything.
And if you’re still scared? Try it on yourself. Take a generic for a month. See how you feel.
Then ask yourself: Why are we making this so hard?
RAJAT KD
January 11, 2026 AT 10:10Generic drugs are bioequivalent. End of story. FDA inspections are rigorous. Manufacturing standards are global. Stop spreading fear.
Darren McGuff
January 12, 2026 AT 07:04I work in a rural UK clinic. We’ve been using generics for decades. The cost savings? Massive. The outcomes? Identical. I’ve had patients switch from brand-name statins to generics - and their LDL didn’t budge. Same side effects. Same efficacy.
The real issue? Patient trust. And that’s built by communication, not legislation.
The FDA toolkit? Brilliant. Simple. Practical. Why aren’t we using it everywhere? It’s not about resources - it’s about will.
Also - authorized generics are the secret weapon. Most docs don’t know they exist. They’re literally the brand drug in a generic box. Zero risk. Max savings. Use them.
Matthew Maxwell
January 13, 2026 AT 06:45It’s embarrassing. A profession built on evidence, yet still clinging to brand-name superstition like it’s 1995.
You think generics are ‘inferior’? Then why do 90% of prescriptions fill them? Why do Medicare, VA, and Kaiser all mandate them? Why do the same manufacturers produce both brand and generic versions in the same facility?
This isn’t ignorance - it’s willful denial. And it’s costing lives. Patients skipping doses. Skipping meals. Choosing between rent and refills.
Stop hiding behind ‘exceptions.’ The exceptions are rare. The harm from inaction is epidemic.
If you’re still prescribing brand-name metformin over generic? You’re not a doctor. You’re a liability.
Lindsey Wellmann
January 14, 2026 AT 21:31OMG I just printed the infographic and taped it to my EHR screen 😭
AND MY PATIENT JUST SAID ‘I DIDN’T KNOW THEY WERE THE SAME’ AND THEN GAVE ME A HUG 🥹💖
Also, I started using the script: ‘Same medicine. Different price.’
Now they ask for generics. Like, on purpose. Like, they’re excited.
Who knew a stapler and a printer could change the world? 🙌
PS: I just sent this to my entire clinic. We’re all doing it now. #GenericsAreGreat
Ashley Kronenwetter
January 15, 2026 AT 09:15As a physician who has spent 18 years in primary care, I can confirm that the data presented here is accurate and actionable. The FDA’s resources are not merely supplementary - they are essential. The integration of these tools into EHRs is not a luxury; it is a clinical imperative.
While I acknowledge the concerns raised by some colleagues regarding individual variability, the overwhelming body of evidence supports generic use as the standard of care. The ethical obligation to reduce financial toxicity outweighs anecdotal apprehension.
I have implemented the Prescriber Flyer in my practice since March 2022. Generic prescribing increased from 64% to 87%. Patient satisfaction scores rose. No adverse events attributable to generics were documented.
It is time for the profession to move beyond myth and embrace evidence. The tools are here. The time is now.