Why generic medicine packaging can’t be trusted at a glance
You pick up a bottle of generic metformin at your pharmacy. It looks right. The label matches the brand. The pills are the same color and shape. But what if it’s fake? Counterfeit generic drugs aren’t rare-they’re everywhere. The World Health Organization estimates that 1 in 10 medicines worldwide are fake, and in some countries, that number jumps to 1 in 3. Generic drugs are especially vulnerable because they’re cheaper, less regulated in some places, and often lack the security features branded drugs have. But that doesn’t mean you’re powerless. Verifying authenticity isn’t just for pharmacists-it’s something anyone who takes generics should know how to do.
What to look for: Overt security features you can check yourself
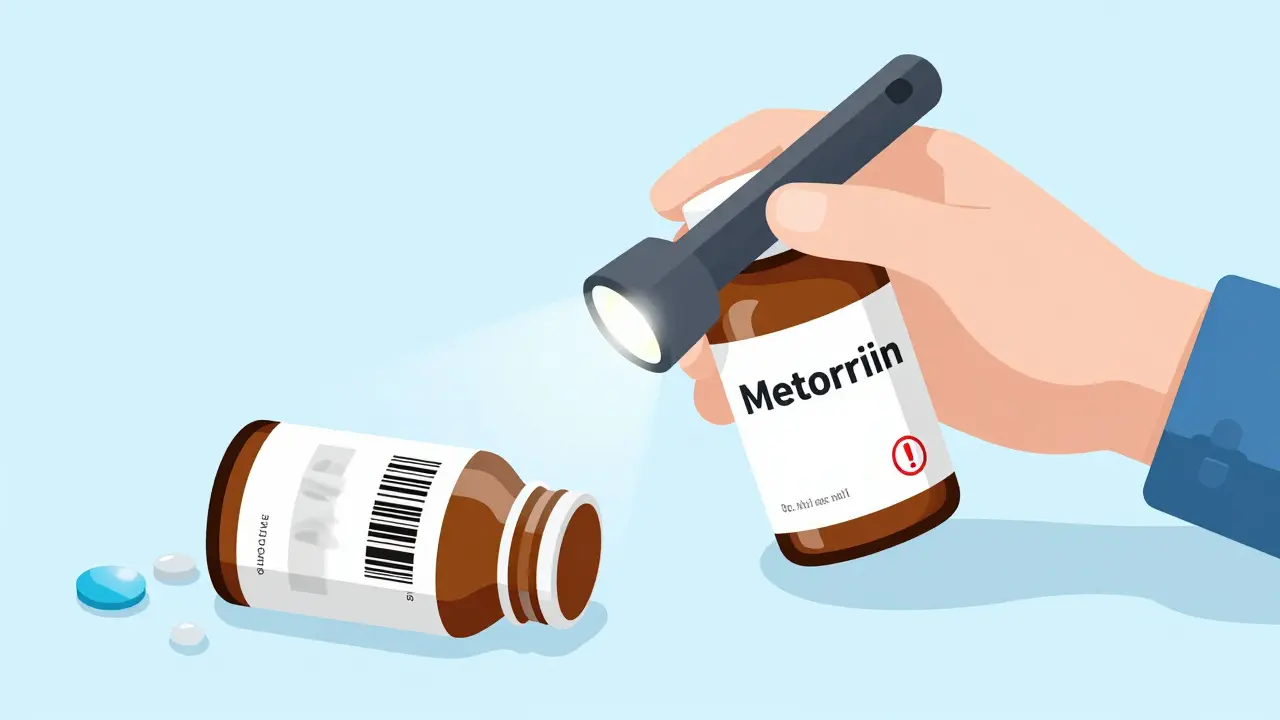
Every legitimate medicine package has visible clues. These are called overt features-things you can see without tools. Look for color-shifting ink. Pfizer’s Viagra packaging, for example, changes from green to blue when tilted. If your generic version doesn’t shift at all, or shifts the wrong way, that’s a red flag. Holograms are another common feature. Real ones have tiny text or images that move as you tilt the box. Counterfeiters copy them, but they often look blurry or flat. Check the font. One pharmacist on Reddit spotted a fake Nexium® because the expiration date used a slightly different font size. It took three tries to notice. That’s how subtle it gets.
Also check the barcode. It should be crisp, not smudged. The numbers should align perfectly with the printed text. If the barcode looks faded or doesn’t scan on your phone, don’t take the risk. Even small things like the texture of the paper or the way the cap seals can be off. Real packaging has consistent pressure seals. Fakes often have uneven glue lines or loose caps.
Covert features: Tools that reveal what the eye can’t see
Many generics now include hidden security features. These need simple tools to detect. A $10 UV flashlight (365nm wavelength) can reveal invisible ink. Johnson & Johnson uses this on Tylenol packaging-authentic bottles glow under UV light. If yours doesn’t, it’s suspect. Some packages have micro-text printed in lines too small to read with the naked eye. You’ll need a 10x magnifier to spot them. These are hard to replicate without expensive equipment.
RFID tags and chemical markers are used by larger manufacturers. These aren’t visible, but handheld readers can detect them. While most consumers won’t have these devices, pharmacists in clinics and hospitals often do. If you’re unsure, ask your pharmacist to check with a UV light or magnifier. Most community pharmacies keep basic verification tools on hand. Don’t be shy-this is your health on the line.
Track-and-trace systems: The digital fingerprint of your medicine
Since 2023, U.S. law requires every prescription medicine to have a unique serial number, following the GS1 standard. This is part of the Drug Supply Chain Security Act (DSCSA). The number is printed as a 2D barcode on the box. Scanning it should pull up verification data from the manufacturer. But here’s the catch: many generic manufacturers don’t fully comply yet. A 2023 report from the Generic Pharmaceutical Association found that 35-45% of generic products still lack consistent serialization. That means even if you scan the barcode, it might not work. Apps like MediMark claim to verify generics, but they fail 40-50% of the time because the data isn’t there.
In the European Union, the system works better. Since 2019, the Falsified Medicines Directive (FMD) requires every prescription drug to be scanned before sale. The European Medicines Verification System handles over 2.5 billion verifications a year with 99.998% uptime. In the U.S., it’s still patchy. If you’re in a state with a regional verification center, you can send your medicine’s serial number for manual check. Some manufacturers like Pfizer offer free verification portals where you can enter the code and get a response within 24 hours.

Spectroscopy: The science behind the truth
Here’s where things get serious. Even if the packaging looks perfect, the pills inside could be fake. That’s where spectroscopy comes in. Devices like the Thermo Fisher TruScan® RM and B&W Tek NanoRam® use infrared or Raman light to analyze the chemical makeup of a pill. They don’t need to open the bottle-just point and scan. These machines detect differences in coating, moisture, or active ingredients that are invisible to the eye. One study found a fake metformin tablet that looked identical to the real one. The NanoRam® flagged it immediately. The pharmacist saved a patient from kidney damage.
But these devices cost $15,000 to $50,000. They’re not for home use. Still, if you’re a pharmacist, hospital worker, or even a patient on long-term therapy, you should know which facilities have them. A 2023 survey of pharmacists found that 94% who used these tools were satisfied. They’re the gold standard for confirming authenticity. The FDA’s Product Quality Research Institute says any verification system must be at least 95% accurate. Spectroscopy hits that mark. Visual checks alone? Only 60-70% reliable.
Why generics are harder to verify than brand-name drugs
Branded drugs get better security. Why? Because they’re profitable. Generic manufacturers operate on thin margins. They spend less on packaging, fewer security features, and often skip track-and-trace systems to cut costs. A 2022 McKinsey report found that implementing full verification adds $500,000 to $2 million per product line. For a small generic maker, that’s a lot. So they cut corners. The result? A 2022 National Community Pharmacists Association survey found that 68% of pharmacists had more trouble verifying generics than branded drugs. The top two reasons? Inconsistent security features (84%) and no reference samples to compare against (76%).
Even when generics are real, their packaging changes often. One batch might have a different label design than the last. That’s normal for generics-manufacturers switch suppliers. But it makes verification harder. If you’re used to one look, a new version might seem suspicious when it’s not. Always check with your pharmacist if something looks off, even if it’s just different.
What you can do right now to protect yourself
- Inspect every package-look for color shifts, holograms, font consistency, and barcode quality.
- Use a UV light-buy a $10 one online. Shine it on the label. Real drugs glow in specific spots.
- Ask your pharmacist-they can use magnifiers, UV lights, or even scan the barcode. Most are happy to help.
- Don’t trust online pharmacies-if the price is too good to be true, it probably is. Stick to licensed U.S. or EU pharmacies.
- Report suspicious drugs-contact the FDA’s MedWatch program or your state board of pharmacy. One report can stop a batch from reaching others.
The future: AI, blockchain, and global standards
Change is coming. By January 2025, the EU will require all generic medicines to have 2D codes with cryptographic authentication-harder to fake than today’s barcodes. The FDA is testing blockchain for tracking generics across 4 distribution tiers. Early results show 99.2% accuracy. By 2026, the U.S. aims to serialize 100% of generics. But global coordination is still weak. INTERPOL’s 2023 Operation Pangea found counterfeiters are now replicating security features with 90-95% accuracy. That means the next wave of fakes won’t be caught by UV lights or holograms alone.
The solution? Layered verification. Use visual checks, then UV, then scan the code, and if possible, use spectroscopy. No single method is perfect. But together, they’re powerful. Experts agree: the future is AI-powered systems that combine all these methods automatically. Gartner predicts 70% of verification will use AI by 2028. Until then, stay alert. Your life might depend on it.
Common questions about generic medicine verification
How can I tell if my generic medicine is fake just by looking at it?
You can spot some fakes by checking for color-shifting ink, holograms that don’t move properly, smudged or misaligned barcodes, and inconsistent font sizes on labels. Even small differences-like the shape of a letter or the thickness of a line-can indicate a counterfeit. Always compare your bottle to the manufacturer’s official images online or ask your pharmacist for a reference sample.
Can I use my phone to scan a generic medicine’s barcode and verify it?
Sometimes, but not reliably. Many generic medicines still lack proper serialization, so scanning the barcode often returns no data. Apps like MediMark work better with branded drugs. For generics, scanning might give you a false sense of security. Always combine scanning with visual checks and ask your pharmacist to verify using UV light or other tools.
Are counterfeit generics dangerous?
Extremely. Fake generics may contain no active ingredient, wrong dosage, toxic chemicals, or contaminants. In 2012, a counterfeit steroid caused a meningitis outbreak in the U.S. that killed 64 people. Even low-dose counterfeits can lead to treatment failure, antibiotic resistance, or organ damage. If you suspect a fake, stop taking it immediately and contact your doctor.
Why don’t all generic manufacturers use advanced security features?
Cost. Generic drugs make up 90% of prescriptions in the U.S. but only 22% of pharmaceutical spending. Manufacturers operate on thin margins and often skip expensive security features like RFID tags or spectroscopy-ready packaging to keep prices low. Regulatory pressure is increasing, but compliance varies widely-especially outside the EU and U.S.
What should I do if I find a fake generic medicine?
Don’t return it to the pharmacy. Contact the FDA’s MedWatch program at 1-800-FDA-1088 or file a report online. Save the packaging, take photos, and note where you bought it. Your report helps the FDA track fake drug networks. If you’re a pharmacist, alert your state board of pharmacy. One report can prevent harm to dozens of people.
What’s next: How to stay ahead of counterfeiters
Keep learning. The fight against fake drugs is ongoing. New counterfeits appear every year. Stay informed through the FDA’s website and your pharmacist’s updates. If you’re on long-term medication, consider joining a patient advocacy group that tracks drug safety. Support policies that fund verification for generics. And never assume a drug is safe just because it’s cheap or looks right. Your vigilance matters more than you think.


 Medications
Medications



Sarah Mailloux
January 17, 2026 AT 04:17Amy Ehinger
January 18, 2026 AT 21:09Ayush Pareek
January 19, 2026 AT 05:51Nilesh Khedekar
January 20, 2026 AT 08:19Nicholas Urmaza
January 20, 2026 AT 20:38Nat Young
January 22, 2026 AT 20:19Jan Hess
January 23, 2026 AT 16:34Iona Jane
January 25, 2026 AT 15:03Jaspreet Kaur Chana
January 27, 2026 AT 12:12Diane Hendriks
January 28, 2026 AT 22:55Annie Choi
January 30, 2026 AT 00:36Nishant Garg
January 31, 2026 AT 08:08