Why Controlled Substance Storage Matters More Than Ever
Every year, tens of thousands of prescription painkillers, sedatives, and stimulants go missing from hospitals, clinics, and pharmacies-not because of theft by outsiders, but because of internal diversion. Someone with access is taking them. Maybe it’s a nurse who’s addicted. Maybe it’s a technician who sells them on the street. Either way, the result is the same: patients don’t get the medicine they need, and lives are put at risk.
The Controlled Substances Act doesn’t just regulate who can prescribe these drugs-it demands how they’re stored. The DEA requires every facility handling Schedule II-V medications to have "effective controls and procedures to guard against theft and diversion." It’s not optional. In 2023, the average civil penalty for failing to secure controlled substances was $187,500. And that’s just the fine. If a patient gets infected because a diverted syringe was reused? Costs jump to over $287,000 per incident.
What Counts as a Controlled Substance?
Not all pain meds are created equal. The DEA classifies them into five schedules based on abuse potential and medical use. Schedule II drugs like oxycodone, fentanyl, and morphine have the highest risk of addiction and are tightly controlled. Schedule III includes hydrocodone with acetaminophen and ketamine. Schedule IV covers benzodiazepines like diazepam and alprazolam. Schedule V includes cough syrups with low-dose codeine.
Here’s the key: every one of these must be stored securely. Even if your state law doesn’t require it for Schedule IV or V drugs, the ASHP Guidelines and DEA best practices say you should. Why? Because diversion doesn’t care about the schedule. A nurse who steals a vial of fentanyl is just as dangerous as one who steals a bottle of lorazepam.
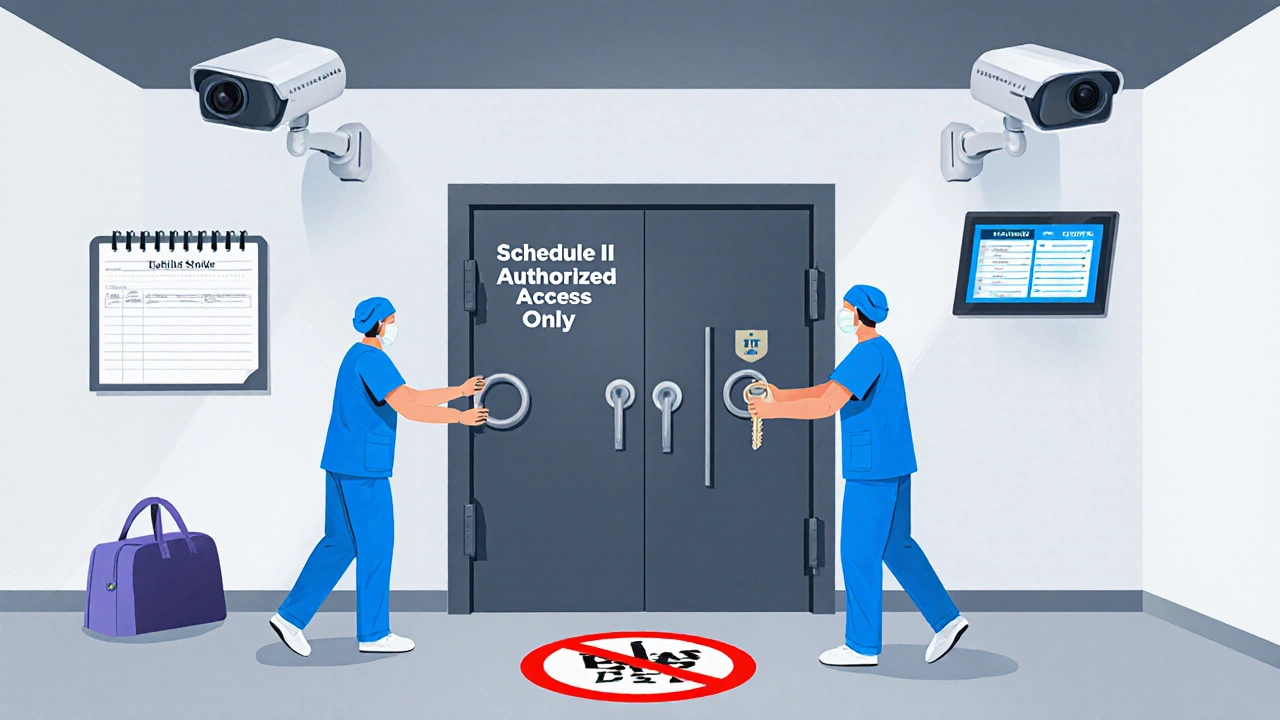
Physical Storage: Locks, Cameras, and Access Limits
Storage isn’t just about having a locked cabinet. It’s about control. The DEA and ASHP agree: access should be limited to one or two people. That means no more than two staff members have keys or codes to the main storage vault.
Here’s what works:
- Use a dedicated, locked vault with a solid door, not just a cabinet in the pharmacy. The vault should be bolted to the floor or wall.
- Install surveillance cameras pointing directly at the vault door and any access points. Footage must be retained for at least 90 days.
- Never allow personal bags, purses, or coats near medication storage areas. In 31% of diversion cases, stolen drugs were hidden in personal items.
- Position lockers and storage units so they’re always visible. No hidden corners. No blind spots.
Small clinics might think they don’t need fancy gear. But a simple, locked, dual-key vault with a logbook signed by two people every time it’s opened is better than nothing. And if you’re storing more than 10kg of Schedule II drugs annually, you’re now required by the DEA to have real-time inventory tracking-effective January 1, 2025.

Automated Dispensing Cabinets (ADCs): The Gold Standard
If your facility has more than 100 beds, an automated dispensing cabinet (ADC) isn’t just a luxury-it’s a necessity. These are like high-tech ATMs for medications. Staff scan their badge or use biometric login, select the drug, and the system logs exactly who took what, when, and why.
Studies show ADCs reduce diversion incidents by 73%. Why? Because they eliminate manual errors and create a digital trail. Every transaction is recorded. No one can fake a signature. No one can claim they "didn’t know" what happened.
But they’re expensive. A single unit costs between $45,000 and $75,000, plus 15% per year in maintenance. For a small clinic, that’s not feasible. That’s why dual-control protocols still matter. If you can’t afford an ADC, make sure every access to the vault requires two people-both signing a log, both witnessing the removal. No exceptions.
The Biggest Risk Points: Where Diversion Happens
Diversion doesn’t happen in the vault. It happens in the gaps between storage and administration.
Here are the top three risk zones:
- Compounding or preparing doses-When a nurse draws up a vial of fentanyl in a back room, there’s no audit trail. That’s where 68% of large-scale cases occur.
- Transferring drugs from pharmacy to floor stock-If you’re using paper logs to track what’s moved to a nursing station, you’re vulnerable. Electronic tracking is mandatory for high-risk drugs.
- Disposing of unused meds-A common trick is to flush a vial of morphine, then replace it with saline. If no one checks the waste logs, it goes unnoticed.
Fix this by requiring two people to witness every dose preparation and disposal. Use barcode scanning for waste disposal. Document every drop of medication that leaves the vault. And never, ever let one person handle both ordering and dispensing.
Staff Training and Culture: The Human Factor
Technology helps, but people prevent diversion. You can have the best locks and cameras in the world, but if staff are afraid to report suspicious behavior, you’re still at risk.
Successful facilities do three things:
- Run mandatory training every six months-not just a 10-minute PowerPoint. Show real cases. Talk about signs of addiction. Explain the legal consequences.
- Create a culture where reporting isn’t seen as "snitching." Use anonymous hotlines. Reward staff who flag odd behavior.
- Review access logs daily. Look for outliers: someone who takes 10 fentanyl doses in a shift when the average is two. Someone who always works the night shift when fewer people are around.
One hospital in Texas reduced diversion by 74% after banning personal bags and requiring dual authentication. But it took three training sessions and six months of consistent enforcement before staff stopped complaining.
What to Do If You Suspect Diversion
If you notice missing drugs, odd patterns in inventory, or a staff member acting strangely-don’t wait. Act immediately.
Here’s your step-by-step:
- Secure the area. Don’t let anyone else access the vault or ADCs until you’ve reviewed logs.
- Review electronic and paper records. Look for unexplained discrepancies, duplicate entries, or missing signatures.
- Alert your pharmacy director and compliance officer. Don’t confront the suspected person yet.
- Report to the DEA within one business day if the loss is significant. Failure to report is a violation.
- Cooperate fully with investigators. They’ll check your storage, your logs, your cameras, your training records.
Remember: the DEA doesn’t just look at the drugs. They look at your procedures. If your policy says two people must be present, but you’ve never enforced it, you’re liable-even if no one stole anything.
Future Trends: AI, Real-Time Tracking, and Rising Pressure
By 2026, the market for diversion prevention tech will hit $1.2 billion. Why? Because regulators are catching up.
AI-powered systems are now being piloted at Mayo Clinic and Johns Hopkins. These tools watch for patterns: a nurse who refills the same ADC every night, or a pharmacy tech who always handles waste disposal after midnight. They cut false alarms by 63% and catch 92% of incidents within 48 hours.
And the pressure is only getting stronger. DEA inspections have risen 37% since 2019. Facilities without proper storage face 4.7 times higher risk of enforcement. The next wave of ASHP guidelines, due in mid-2024, will require all facilities to track saline flushes used to replace stolen drugs.
Ignoring this isn’t negligence. It’s a gamble with patient safety-and your license.
Bottom Line: Security Is a Process, Not a Product
You can’t buy your way out of diversion risk. You can’t just install a lock and call it done. Prevention is a daily practice: checking logs, training staff, watching for changes, enforcing rules without exception.
Start small if you must. But start now. Lock your vault. Limit access. Log everything. Train your team. Report anomalies. The cost of doing nothing isn’t just a fine. It’s a patient who dies because the painkiller they needed was stolen by someone who had access.


 Medications
Medications



Deepali Singh
November 16, 2025 AT 14:22Also, why is the DEA only cracking down now? This has been going on for a decade.
Sylvia Clarke
November 18, 2025 AT 10:57It’s like installing a titanium deadbolt on a house where the front door is held shut with duct tape. The real problem isn’t the lock-it’s the fact that we’ve turned healthcare into a supply chain with soul-crushing turnover and zero accountability.
And yet, we punish the nurse who steals because she’s in pain… while the hospital execs get bonuses for hitting "cost savings" targets.
Someone please tell me how this is ethical.
Jennifer Howard
November 20, 2025 AT 03:40Abdul Mubeen
November 21, 2025 AT 08:34And who benefits? The pharmaceutical companies who want you to believe addiction is a theft problem, not a public health crisis.
Also, why are we not talking about why nurses are stealing? Because they’re overworked, underpaid, and traumatized. But no, let’s just lock them out and call it compliance.
mike tallent
November 21, 2025 AT 11:57It’s low-tech but it works. Staff started calling it "The Photo Rule" and now no one even thinks about stealing.
Also, we banned bags. No more purses. Everyone gets a locker. It’s weird at first but now people say it feels safer. 🛡️💉
Joyce Genon
November 23, 2025 AT 10:53And you want to install AI to track her behavior? Brilliant. Let’s surveil the people who are literally keeping you alive while you sip your $12 latte.
Also, the DEA doesn’t care about your "culture". They care about paperwork. And if your logbook has a smudge? You’re fined. But if a patient dies from a botched pain management because the nurse was too scared to ask for help? That’s just "unfortunate".
John Wayne
November 24, 2025 AT 02:48Furthermore, the suggestion that training can prevent diversion is a fantasy. Human behavior is not modifiable through workshops. It is governed by biology, trauma, and systemic failure.
And yet, here we are, treating a tumor with band-aids.
Julie Roe
November 25, 2025 AT 14:00Locks and cameras won’t fix that. What works? A manager who says, "Hey, you seem off. Want to talk?" A quiet chat over coffee. A leave of absence without punishment. A peer support group that doesn’t feel like a witch hunt.
One hospital I worked at started a "No Shame" hour every Thursday-staff could anonymously drop notes about struggles. We found out three people were using their own meds because they couldn’t get insurance coverage. We fixed it. No one got fired. Everyone got help.
Security isn’t about control. It’s about care. And care doesn’t come from a vault. It comes from a human being who notices someone’s missing.
jalyssa chea
November 26, 2025 AT 06:39Gary Lam
November 27, 2025 AT 17:21Our diversion rate? 0.2%. Yours? 3.8%. Coincidence? I think not.
Also, we don’t call it "diversion". We call it "a cry for help". Try it sometime. It works.
Peter Stephen .O
November 29, 2025 AT 08:00Here’s what I’ve learned in 15 years: the best security system is a team that trusts each other. Not a vault. Not a camera. Not an ADC.
When you treat people like humans-not threats-you get loyalty. When you punish mistakes instead of fixing systems, you get silence.
And yeah, I’m totally pushing for the selfie rule in my unit. It’s weird. It’s funny. It’s human. And it works. 🙌
Andrew Cairney
November 30, 2025 AT 23:01Next thing you know, they’ll implant RFID chips in nurses so they can monitor stress levels before they "steal".
And don’t get me started on AI. It’s not catching diversion-it’s profiling. That nurse who works nights? She’s "anomalous". That tech who takes extra breaks? He’s "high risk".
Meanwhile, the CEO who approved 10% budget cuts to mental health? No one’s auditing him.
This isn’t about safety. It’s about control. And it’s going to get worse.
Just wait till they start using facial recognition to detect "suspicious expressions". 😈